Edited, memorised or added to reading queue
on 30-Sep-2022 (Fri)
Do you want BuboFlash to help you learning these things? Click here to log in or create user.
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
Unknown title
ingenious but unsuccessful fixes finally convinced him that in fact there was no solution: there was no way to arrange a machine to do work for nothing. This lesson stayed with Mayer for life. <span>Mayer studied to become a medical doctor (his studies included one physics course) and in 1840, at age 25, he signed on as a ship’s doctor on a ship bound for the tropics. Shortly after reaching the Dutch East Indies, some of the sailors became ill and Mayer’s treatment included blood letting. He was amazed to find that the venous blood was a bright red, almost the same as arterial blood. Back in Germany, the venous blood was much darker, and there was a reason: the chemist Lavoisier had explained that the body’s use of food, at least in part, amounted to burning it in a controlled way to supply warmth. The darker venous blood in effect contained the ashes, to be delivered to the lungs and expelled as carbon dioxide. Mayer concluded that less burning of food was needed to keep warm in the tropics, hence the less dark blood. Now, Lavoisier had claimed that the amount of heat generated by burning, or oxygenation, of a certain amount of carbon did not depend on the sequence of chemical reactions involved, so the heat generated by blood chemistry oxygenation would be the same as that from uncontrolled old-fashioned burning in air. This quantitative formulation led Mayer to think about how he would measure the heat generated in the body, to equate it to the food burned. But this soon led to a problem. Anyone can generate extra heat, just by rubbing the hands together, or, for example, by turning a rusty, unoiled wheel: the axle will get hot. Does this ‘outside’ heat also count as generated by the food? Presumably yes, the food powers the body, and the body generates the heat, even if indirectly. Mayer was convinced from his childhood experience with the water wheel that nothing came from nothing: that outside heat could not just appear from nowhere, it had to have a cause. But he saw that if the indirectly generated heat must also be included, there is a problem. His analysis ran something like this (I’ve changed the illustration slightly, but the idea’s the same): suppose two people are each steadily turning large wheels at the same rate, and the wheels are equally hard to turn. One of them is our rusty unoiled wheel from the last paragraph, and all that person’s efforts are going into generating heat. But the other wheel has a smooth, oiled axle and generates a negligible amount of heat. It is equally hard to turn, though, because it is raising a large bucket of water from a deep well. How do we balance the ‘food = heat’ budget in this second case? Mayer was forced to the conclusion that for the ‘food = heat’ equation to make sense, there had to be another equivalence: a certain amount of mechanical work, measured for example by raising a known weight through a given distance, had to count the same as a certain amount of heat, measured by raising the temperature of a fixed amount of water, say, a certain number of degrees. In modern terms, a joule has to be equivalent to a fixed number of calories. Mayer was the first to spell out this ‘mechanical equivalent of heat’ and in 1842 he calculated the number using results of experiments done earlier in France on the specific heats of g
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
Unknown title
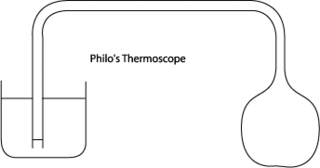
d, how likely is it that a group of people can set a uniform standard? We need to construct a device of some kind that responds to temperature in a simple, measurable way—we need a thermometer. <span>The first step on the road to a thermometer was taken by one Philo of Byzantium, an engineer, in the second century BC. He took a hollow lead sphere connected with a tight seal to one end of a pipe, the other end of the pipe being under water in another vessel. To quote Philo: “…if you expose the sphere to the sun, part of the air enclosed in the tube will pass out when the sphere becomes hot. This will be evident because the air will descend from the tube into the water, agitating it and producing a succession of bubbles. Now if the sphere is put back in the shade, that is, where the sun’s rays do not reach it, the water will rise and pass through the tube …” “No matter how many times you repeat the operation, the same thing will happen. In fact, if you heat the sphere with fire, or even if you pour hot water over it, the result will be the same.” Notice that Philo did what a real investigative scientist should do—he checked that the experiment was reproducible, and he established that the air’s expansion was in response to heat
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
Unknown title
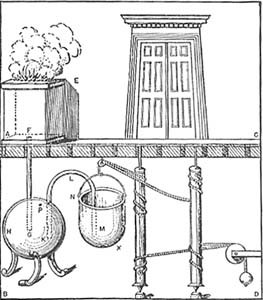
he checked that the experiment was reproducible, and he established that the air’s expansion was in response to heat being applied to the sphere, and was independent of the source of the heat. <span>Some Classic Dramatic Uses of Temperature-Dependent Effects This expansion of air on heating became widely known in classical times, and was used in various dramatic devices. For example, Hero of Alexandria describes a small temple where a fire on the altar causes the doors to open. The altar is a large airtight box, with a pipe leading from it to another enclosed container filled with water. When the fire is set on top of the altar, the air in the box heats up and expands into a second container which is filled with water. This water is forced out through an overflow pipe into a bucket hung on a rope attached to the door hinges in such a way that as the bucket fills with water, it drops, turns the hinges, and opens the doors. The pipe into this bucket reaches almost to the bottom, so that when the altar fire goes out, the water is sucked back and the doors close again. (Presumably, once the fire is burning, the god behind the doors is ready to do business and the doors open…) Still, none of these ingenious devices is a thermometer. There was no attempt (at least none recorded) by Philo or his followers to make a quantitative measurement of how hot or cold th
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
Unknown title
perature change, a thermometer can measure the temperature change.) Galileo used an inverted narrow-necked bulb with a tubular neck, like a hen’s egg with a long glass tube attached at the tip. <span>He first heated the bulb with his hands then immediately put it into water. He recorded that the water rose in the bulb the height of “one palm”. Later, either Galileo or his colleague Santorio Santorio put a paper scale next to the tube to read off changes in the water level. This definitely made it a thermometer, but who thought of it first isn’t clear (they argued about it). And, in fact, this thermometer had problems. Question: what problems? If you occasionally top up the water, why shouldn’t this thermometer be good for recording daily changes in temperature? Answer: because it’s also a barometer! But—Galileo didn’t know about the atmospheric pressure. Torricelli, one of Galileo’s pupils, was the first to realize, shortly after Galileo died, that the real driving force in suction was external atmospheric pressure, a satisfying mechanical explanation in contrast to the philosophical “nature abhors a vacuum”. In the 1640’s, Pascal pointed out that the variability of atmospheric pressure rendered the air thermometer untrustworthy. Liquid-in-glass thermometers were used from the 1630’s, and they were of course insensitive to barometric pressure. Meteorological records were kept from this time, but there was no real uniformity of temperature measurement until Fahrenheit, almost a hundred years later. Newton’s Anonymous Table of Temperatures The first systematic account of a range of different temperatures, “Degrees of Heat”, was written by Newton, but published anonymously, in 1701.
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
Unknown title
ed considerably. He used a linseed oil liquid in glass thermometer up to the melting point of tin (232°C). (Linseed oil doesn’t boil until 343°C, but that is also its autoignition temperature!) <span>Newton tried to estimate the higher temperatures indirectly. He heated up a piece of iron in a fire, then let it cool in a steady breeze. He found that, at least at the lower temperatures where he could cross check with his thermometer, the temperature dropped in a geometric progression, that is, if it took five minutes to drop from 80° above air temperature to 40° above air temperature, it took another five minutes to drop to 20° above air, another five to drop to 10° above, and so on. He then assumed this same pattern of temperature drop was true at the high temperatures beyond the reach of his thermometer, and so estimated the temperature of the fire and of iron glowing red hot. This wasn’t very accurate—he (under)estimated the temperature of the fire to be about 600°C. Fahrenheit’s Excellent Thermometer The first really good thermometer, using mercury expanding from a bulb into a capillary tube, was made by Fahrenheit in the early 1720’s. He got the i
Flashcard 7552550702348
| status | not learned | measured difficulty | 37% [default] | last interval [days] | |||
|---|---|---|---|---|---|---|---|
| repetition number in this series | 0 | memorised on | scheduled repetition | ||||
| scheduled repetition interval | last repetition or drill |
Unknown title
bodies in “thermal contact” eventually come into “thermal equilibrium”—which means they finally attain the same temperature, after which no further heat flow takes place. This is equivalent to: <span>The Zeroth Law of Thermodynamics: If two objects are in thermal equilibrium with a third, then they are in thermal equilibrium with each other. The “third body” in a practical situation is just the thermometer. It’s perhaps worth pointing out that this trivial sounding statement certainly wasn’t obvious before the invention of the thermometer. With only the sense of touch to go on, few people would agree that a piece of wool and a bar of metal, both at 0°C, were at the same temperature. Temperature is a Potential for Heat Flow So this is how to understanding temperature: Black observed that things in thermal contact eventually reach a common temperature, just a connect
bodies in “thermal contact” eventually come into “thermal equilibrium”—which means they finally attain the same temperature, after which no further heat flow takes place. This is equivalent to: <span>The Zeroth Law of Thermodynamics: If two objects are in thermal equilibrium with a third, then they are in thermal equilibrium with each other. The “third body” in a practical situation is just the thermometer. It’s perhaps worth pointing out that this trivial sounding statement certainly wasn’t obvious before the invention of the thermometer. With only the sense of touch to go on, few people would agree that a piece of wool and a bar of metal, both at 0°C, were at the same temperature. Temperature is a Potential for Heat Flow So this is how to understanding temperature: Black observed that things in thermal contact eventually reach a common temperature, just a connect
Flashcard 7552553061644
| status | not learned | measured difficulty | 37% [default] | last interval [days] | |||
|---|---|---|---|---|---|---|---|
| repetition number in this series | 0 | memorised on | scheduled repetition | ||||
| scheduled repetition interval | last repetition or drill |
Unknown title
bvious before the invention of the thermometer. With only the sense of touch to go on, few people would agree that a piece of wool and a bar of metal, both at 0°C, were at the same temperature. <span>Temperature is a Potential for Heat Flow So this is how to understanding temperature: Black observed that things in thermal contact eventually reach a common temperature, just a connected bodies of water will come to the same level if there is no exterior source or sink. In other words, in analyzing heat flow, we can understand temperature as a sort of potential. Just as gravitational potential difference will cause water to flow between connected lakes at different levels, temperature difference between thermally connected objects causes heat to flow. Books I used in preparing this lecture: A Source Book in Greek Science, M. R. Cohen and I. E. Drabkin, Harvard university Press, 1966. A History of the Thermometer and its Uses in Meteo
bvious before the invention of the thermometer. With only the sense of touch to go on, few people would agree that a piece of wool and a bar of metal, both at 0°C, were at the same temperature. <span>Temperature is a Potential for Heat Flow So this is how to understanding temperature: Black observed that things in thermal contact eventually reach a common temperature, just a connected bodies of water will come to the same level if there is no exterior source or sink. In other words, in analyzing heat flow, we can understand temperature as a sort of potential. Just as gravitational potential difference will cause water to flow between connected lakes at different levels, temperature difference between thermally connected objects causes heat to flow. Books I used in preparing this lecture: A Source Book in Greek Science, M. R. Cohen and I. E. Drabkin, Harvard university Press, 1966. A History of the Thermometer and its Uses in Meteo
Flashcard 7552565382412
| status | not learned | measured difficulty | 37% [default] | last interval [days] | |||
|---|---|---|---|---|---|---|---|
| repetition number in this series | 0 | memorised on | scheduled repetition | ||||
| scheduled repetition interval | last repetition or drill |
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
| status | not read | reprioritisations | ||
|---|---|---|---|---|
| last reprioritisation on | suggested re-reading day | |||
| started reading on | finished reading on |
 Some Classic Dramatic Uses of Temperature-Dependent
Effects
Some Classic Dramatic Uses of Temperature-Dependent
Effects  clear (they argued about it). And, in fact, this
thermometer had problems.
clear (they argued about it). And, in fact, this
thermometer had problems.